The US has approved Pfizer’s Covid 19 pill for home use. The company says that this pill is beneficial for corona patients and is also effective on the new variant. Pfizer Inc. said on Wednesday that the US Food and Drug Administration has approved an antiviral COVID-19 pill. This will make it the first home remedy for corona virus. It is claimed that this pill prevents the virus from spreading rapidly.
According to the agency, data from Pfizer’s clinical trials showed that its two-drug antiviral regimen was effective in patients with severe disease. 90% effective in preventing hospitalization and death. Recent lab data suggests that the drug is effective against the Omicron variant as well. This drug can be used to treat more severe patients and patients who are at least 12 years of age.
The company said that it is ready to start immediate deliveries in the US. It is set to increase its production from 80 million to 120 million in 2022. The US government has contracted for 10 million doses of the drug Pfizer. It is priced at $530 per course.
The old antiviral drug, Pfizer’s tablets, will be sold under the brand name Paxlovid. These tablets are to be taken every 12 hours for five days immediately after the onset of symptoms of corona. American citizens will be able to take it at home to avoid the effects of the corona virus. Work on this was going on for a long time. Paxlovid is an inexpensive way to treat early COVID-19 infections. Initial supplies will be extremely limited. An antiviral pill from Merck is also expected to be approved soon.
The Food and Drug Administration has approved the use of Pfizer’s drug for people 12 years of age and older. Children must be at least 88 pounds (40 kg) to take the medicine. Pfizer says it is creating 80 million courses globally next year under contracts with the UK, Australia and other countries.
The FDA gave this approval on the results of the company’s 2,250-patient trial. In this, less than 1% of patients who took the drug were hospitalized within three days of symptoms. No one died at the end of the 30-day research.

![Visa and Social Media [TKB World]](https://topknowledgebox.com/iphaphoo/2025/12/15122025.jpg)
![Vishwakarma Puja 2025 [TKB- INDIA]](https://topknowledgebox.com/iphaphoo/2025/09/17092025-150x150.jpg)
![Ganesh Chaturthi 2025[TKB-INDIA]](https://topknowledgebox.com/iphaphoo/2025/08/27082025-150x150.jpg)
![Buddha Purnima 2025 [TKB INDIA]](https://topknowledgebox.com/iphaphoo/2025/05/12052025-150x150.jpg)



More Stories
Visa and Social Media [TKB World]
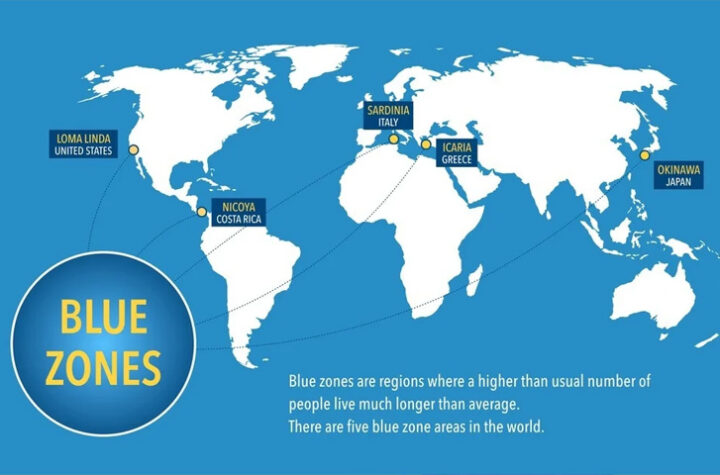
Why do people living in blue zones live longer? 3 reasons that will change your thinking
Children born from today (01/01/2025) will be Generation BETA… Know why ?